Notable efficacy for an off-the-shelf immunotherapy
TECVAYLI (teclistamab) demonstrated notable efficacy for an off-the-shelf immunotherapy, with 59.4% of patients achieving VGPR or better
- After 23 months of follow-up, ORR was 63%
- 45.5% patients achieved ≥CR
- 81.5% of MRD evaluable patients achieve MRD negativity at any point
- The median time to first response was 1.2 months (range: 0.2–5.5), and the median time to achieved ≥CR was 4.6 months (range: 1.6-18.5)
Overall response rate
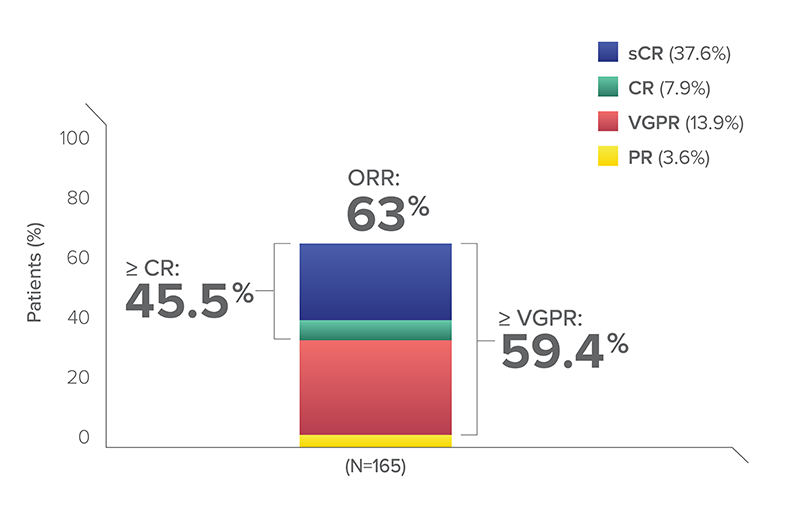
ORR across patient populations
Efficacy was consistent regardless of cytogenetic risk or extent of prior therapy refractoriness
Adapted from Moreau et al. 2022
*del(17p), t(4;14) and/or t(14;16)
**≥1 PI, ≥1 IMiD and ≥1 anti-CD38 mAb
†≥2 PI, ≥2 IMiD and ≥1 anti-CD38 mAb
^Median follow up of 14.1 months
Progression-free survival
Median FU:23 months
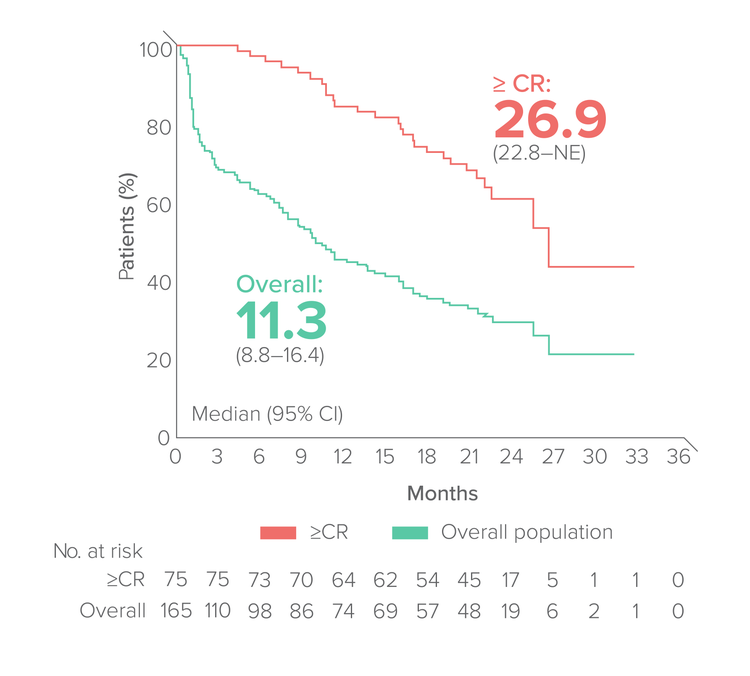
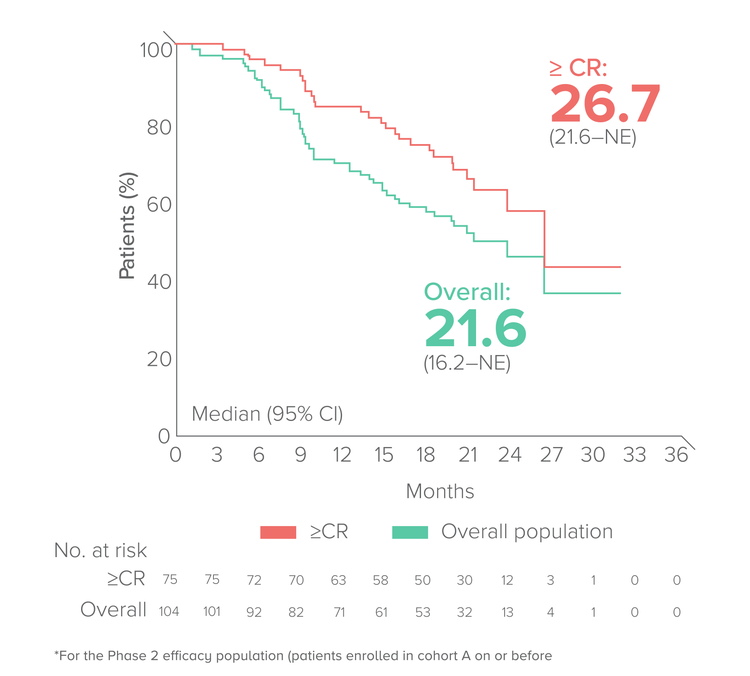
Duration of response
Median FU:23 months
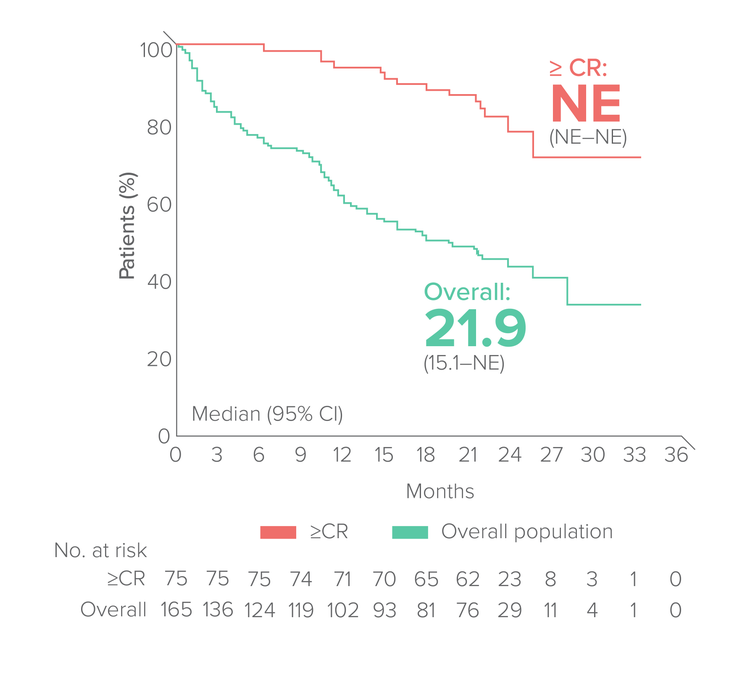
Overall survival
Median FU:23 months

For further information regarding TECVAYLI including full indications, all adverse effects and data please refer to the Israeli MOH prescribing information: https://israeldrugs.health.gov.il/#!/byDrug
The data in this presentation is based on published clinical studies, please see references at the bottom of the slides/throughout the presentation.
CP-412257 - November 2023