Guidelines For The Treatment Of
Pulmonary Arterial Hypertension (PAH)
According to the 2015 ESC/ERS guidelines on pulmonary hypertension, the overall treatment goal in patients with PAH is to achieve or maintain a low-risk status, which is usually associated with good exercise capacity, good quality of life, good right ventricle function and a low mortality risk. Patients at low risk have been shown to have the best outcomes.
Regular comprehensive risk assessment of PAH patients is recommended to evaluate patients’ risk status and escalate therapy if required.
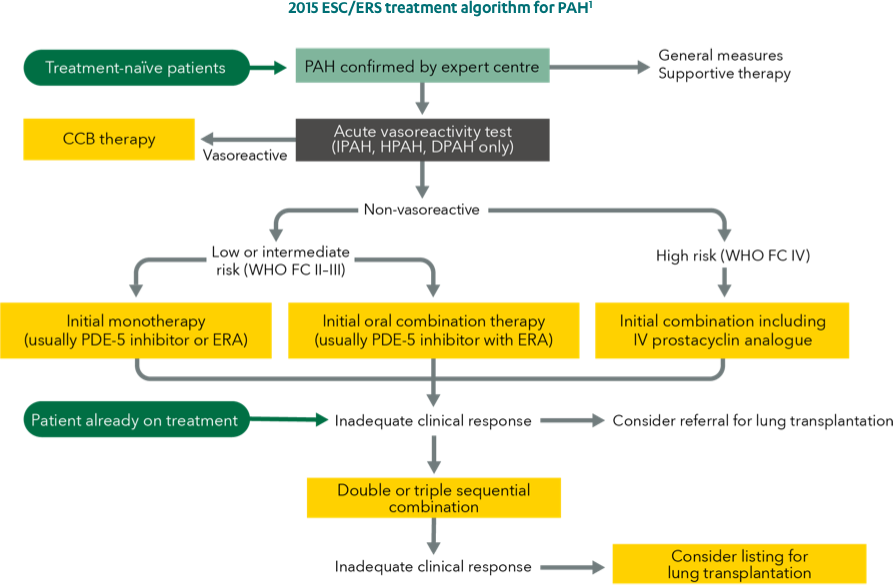
Adapted from Galiè et al. 2016
CCB, calcium channel blocker; DPAH, drug-induced pulmonary arterial hypertension; ERA, endothelin receptor antagonist; ERS, European Respiratory Society; ESC, European Society of Cardiology; FC, functional class; HPAH, heritable pulmonary arterial hypertension; IPAH, idiopathic pulmonary arterial hypertension; IV, intravenous; PAH, pulmonary arterial hypertension; PDE-5, phosphodiesterase type 5; WHO, World Health Organization
Escalation to triple combination therapy is recommended when the initial treatment approach with double combination therapy results in an intermediate risk status.
UPTRAVI® is the only drug with a class I recommendation for early sequential combination with an ERA and/or a PDE-5i.
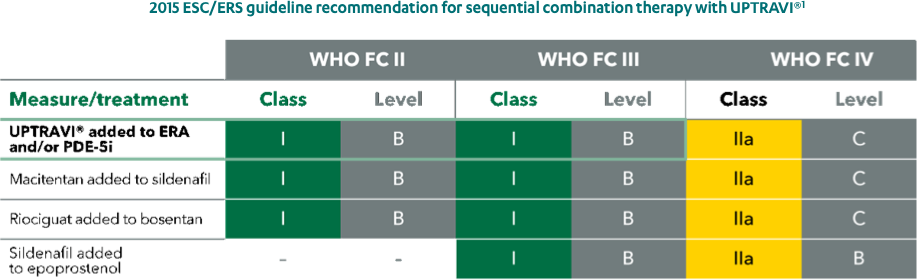
Adapted from Galiè et al. 2016
ERA, endothelin receptor antagonist; FC, functional class; PDE-5i, phosphodiesterase type-5 inhibitor; WHO, World Health Organization
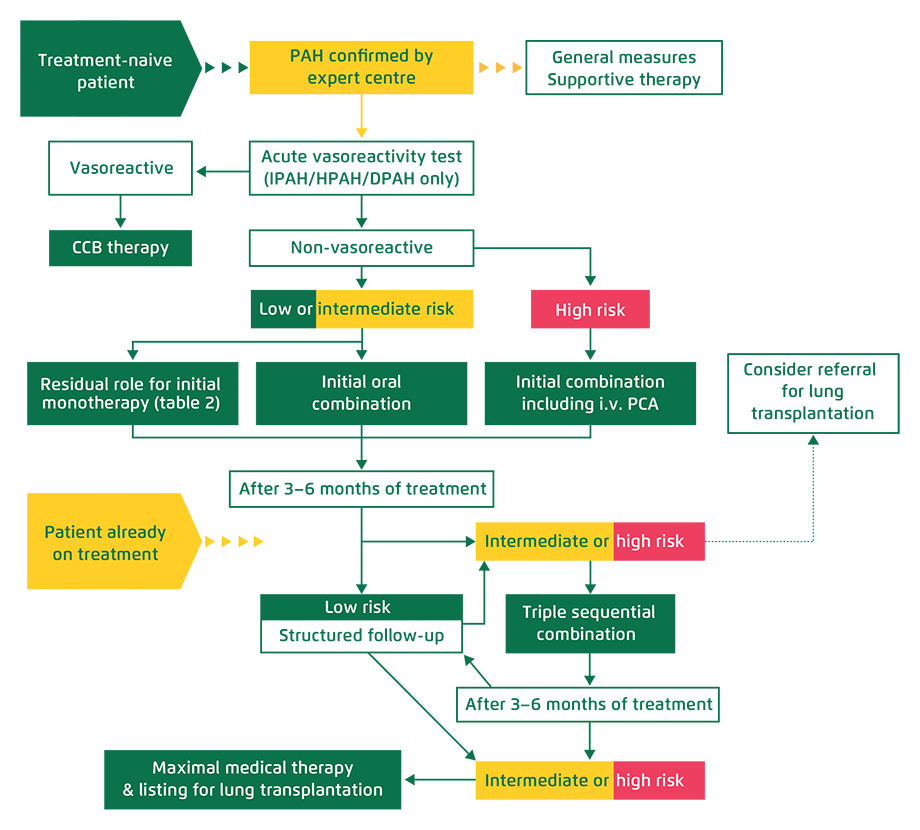
Treatment Algorithm (2018)
WORLD SYMPOSIUM ON PULMONARY HYPERTENSION
Learn More About Uptravi®

Patient Management
Advice on how to manage your UPTRAVI® patients, including details on dosage and side effects.

Product Overview
A synopsis of the key benefits of UPTRAVI® in the treatment of PAH.