Pivotal trial data: GRIPHON
Defend patients against PAH progression with UPTRAVI®
In the GRIPHON trial, UPTRAVI® (selexipag) demonstrated:
- 40% risk reduction in morbidity/mortality events vs placebo (p<0.001)*
- 33% risk reduction in PAH-related hospitalisation vs placebo (p=0.04)*
Time to first disease progression event
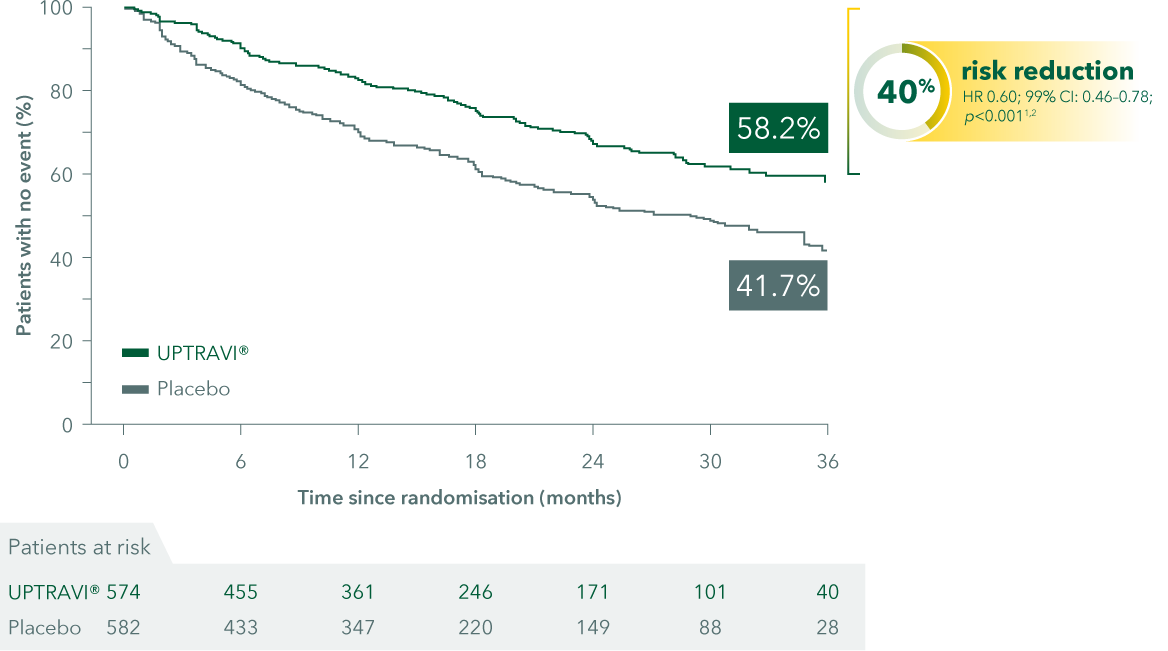
Adapted from Sitbon et al. 2015 and UPTRAVI® PI
In the GRIPHON trial:

patients
randomised

median duration of
UPTRAVI® use

mean time from PAH diagnosis
in patients on UPTRAVI®

patients enrolled within
6 months of diagnosis
*Morbidity/mortality events with UPTRAVI® vs placebo: 27.0% vs 41.6% (HR 0.60; 99% CI: 0.46–0.78; p<0.001, primary endpoint).
PAH-related hospitalisation with UPTRAVI® vs placebo: 13.6% vs 18.7% (HR 0.67; 99% CI: 0.46–0.98; p=0.04).
GRIPHON study design
GRIPHON is the longest and largest PAH trial to date
- A multicentre, double-blind, randomised, parallel-group, placebo-controlled, event-driven Phase 3 trial assessing the effect of UPTRAVI® on time to first morbidity or mortality event*
- Recruited a broad range of patients, including those with CTD-PAH and CHD-PAH
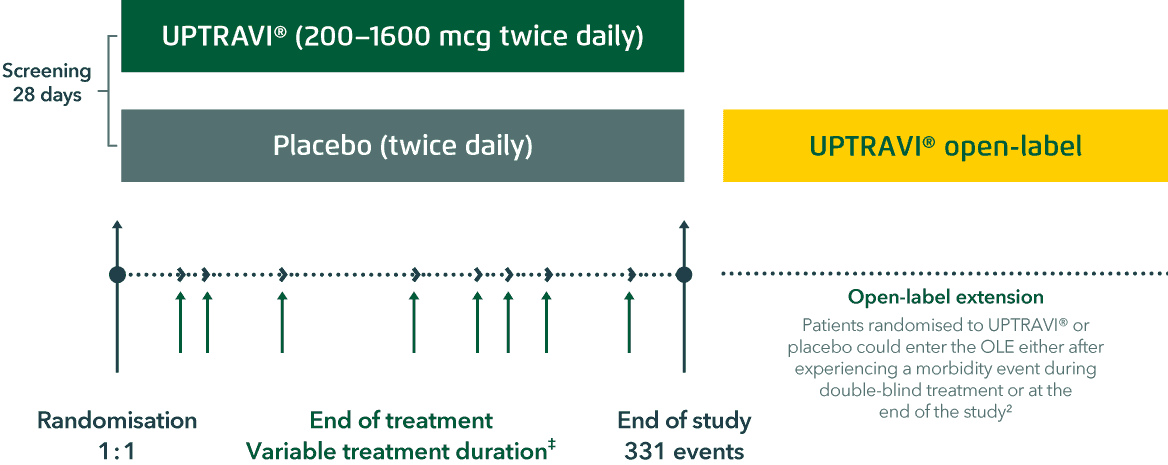
Based on Sitbon et al. 2015 and Galiè et al. 2022
*The primary endpoint was a composite of death or a complication related to PAH, whichever occurred first, up to the end of the treatment period. Complications related to PAH were disease progression or worsening of PAH that resulted in hospitalisation, initiation of parenteral prostanoid therapy or long-term oxygen therapy, or the need for lung transplantation or balloon atrial septostomy – as judged by the physician. Results do not apply to mortality on its own.
‡End of the treatment period was defined for each patient as 7 days after the last intake of study treatment.
Early UPTRAVI® initiation provides a more pronounced risk reduction vs delayed use
Early UPTRAVI® initiation (within 6 months of diagnosis) provides a more pronounced reduction in risk of morbidity/mortality vs placebo compared to delayed use (55% vs 26%).
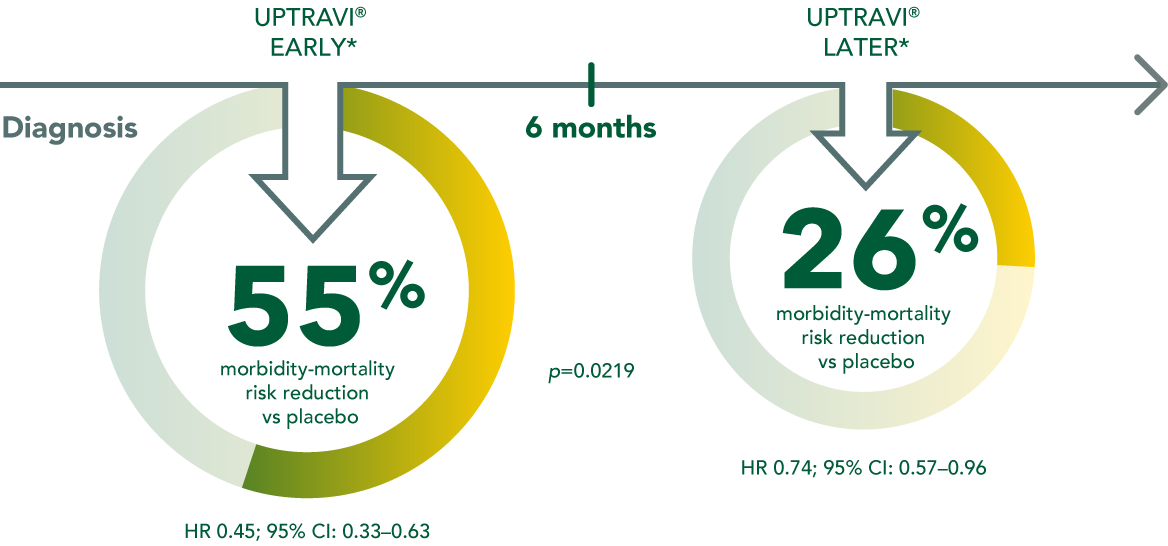
Based on Gaine et al. 2021
*Early: ≤6 months from diagnosis, later: >6 months from diagnosis.
Real-world evidence: SPHERE
UPTRAVI® has proven benefits in the real-world setting
SPHERE is a US-based, multicentre, prospective, real-world, observational drug registry of adult patients initiating UPTRAVI® who were followed for ≤18 months.
Estimated 18-month survival rates were 89.4%, 84.2% and 94.5% in the overall, newly initiated and previously initiated patient populations, respectively. Patients were defined as newly or previously initiated if they had started UPTRAVI® ≤60 days or >60 days, respectively, before enrolment.
The data suggest a more pronounced survival benefit for patients who were initiated on UPTRAVI® earlier* vs later in SPHERE.
Among the 759 patients with PAH, the median duration of UPTRAVI® titration was 8.1 weeks and median maintenance dose was 1100 mcg twice daily.
Overall, previously initiated, newly initiated 18-month survival
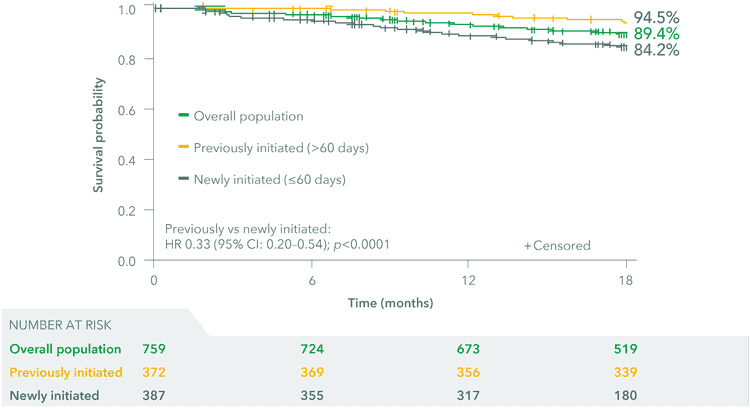
Adapted from McLaughlin et al. 2024
*Patients were defined as newly or previously initiated if they had started UPTRAVI® ≤2 months or >2 months, respectively, before enrolment.
Learn more about UPTRAVI®

Guidelines recommend early selexipag (UPTRAVI®) addition at first follow-up for patients at intermediate-low risk on double therapy
UPTRAVI® has a predictable and manageable long-term safety profile backed by 10 years’ follow-up
Flexible up-titration of UPTRAVI® to help optimise outcomes
Abbreviations
CHD, congenital heart disease; CI, confidence interval; CTD, connective tissue disease; HR, hazard ratio; OLE, open-label extension; PAH, pulmonary arterial hypertension
For further information regarding Opsumit® (macitentan) or Uptravi® (Selexipag) including full indications, all adverse effects and data please refer to the Israeli MOH prescribing information: https://israeldrugs.health.gov.il/#!/byDrug
The data in this presentation is based on published clinical studies, please see references at the bottom of the page.