The safety and tolerability of UPTRAVI® were investigated in the GRIPHON trial, the largest outcomes trial conducted in pulmonary arterial hypertension (PAH) to date. The majority of adverse events (AEs) in the trial were mild to moderate and manageable with treatment. Additionally, prostacyclin pathway-associated AEs were less frequent after the individualised maintenance dose was reached.
Prostacyclin-associated AEs
Most common AEs are typical of drugs that activate the prostacyclin pathway.
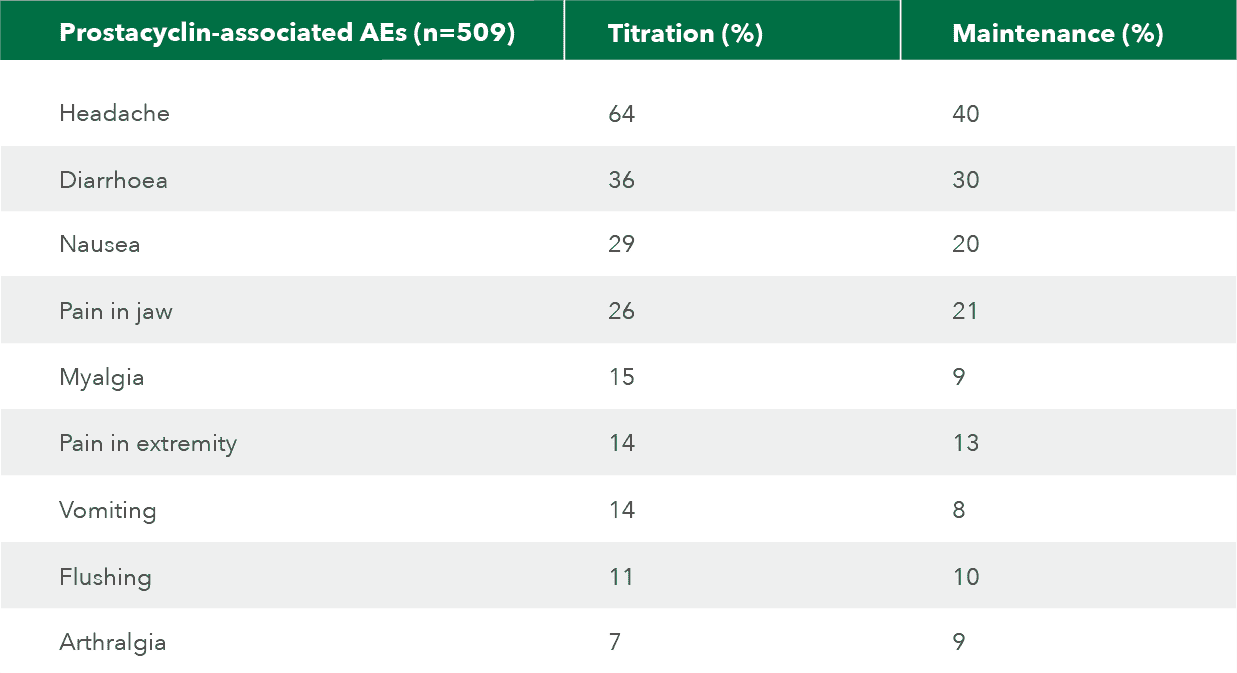
Adapted from Uptravi Israel PI, 7/2022
Adverse events
Overall, 14% of patients in the UPTRAVI® group and 7% of patients in the placebo group prematurely discontinued treatment due to an AE in GRIPHON. The majority of AEs were mild to moderate and manageable with treatment.
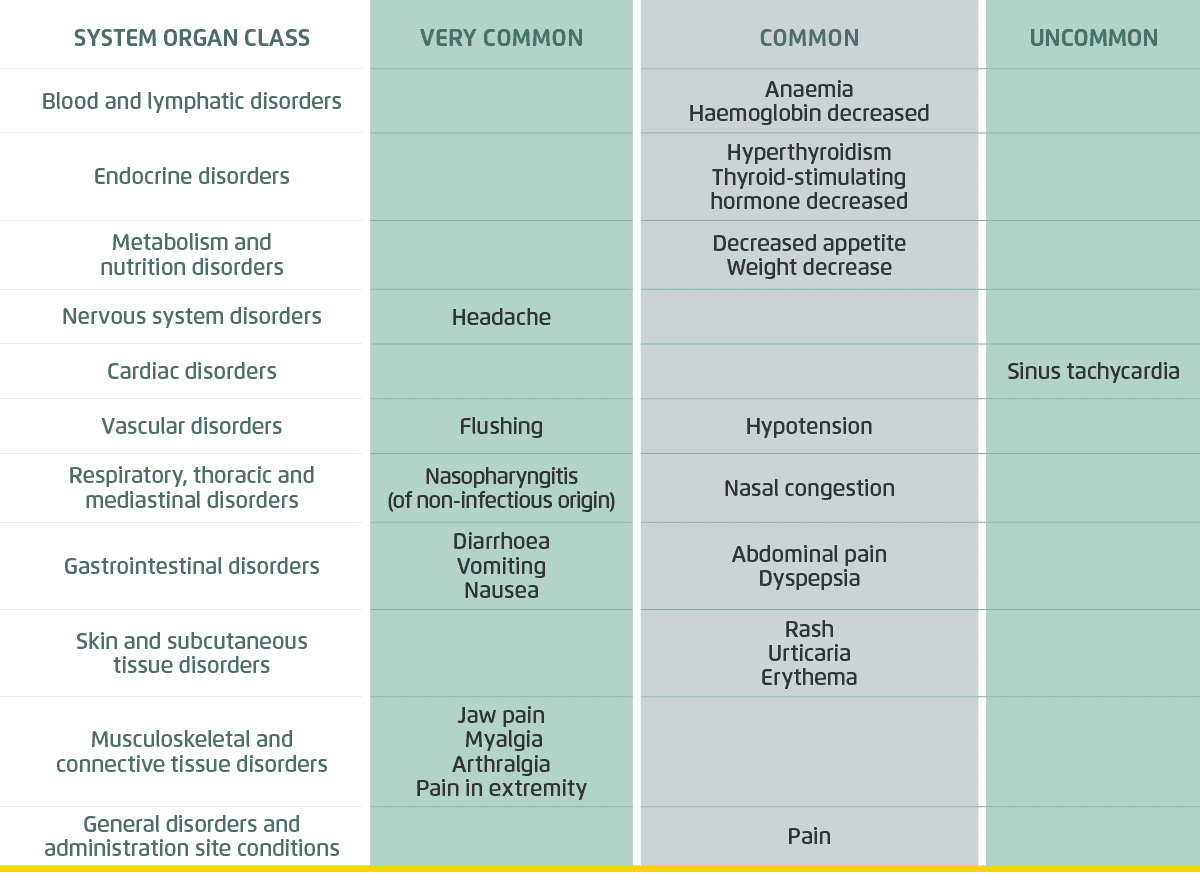
Adapted from Uptravi Israel PI.
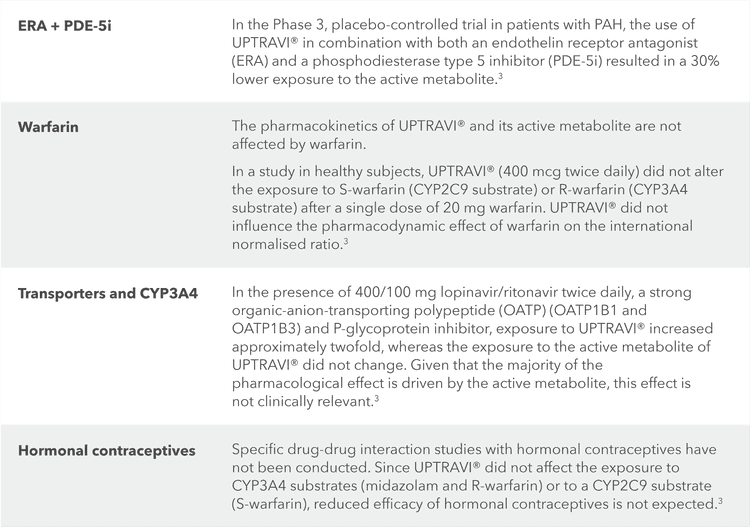
Drug-drug interactions
For further information regarding Opsumit® (macitentan) or Uptravi® (Selexipag) including full indications, all adverse effects and data please refer to the Israeli MOH prescribing information: https://israeldrugs.health.gov.il/#!/byDrug
The data in this presentation is based on published clinical studies, please see references at the bottom of the page.